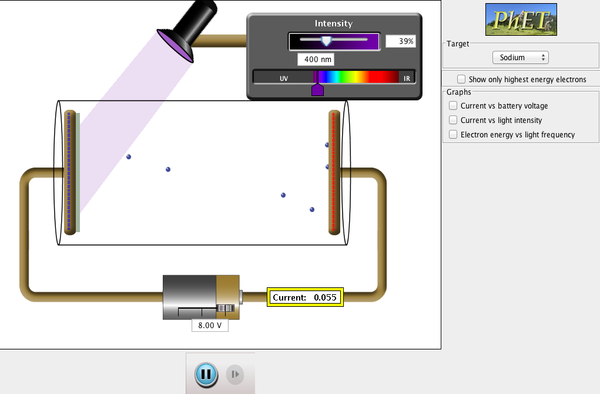
Launch the Photoelectric Effect simulation (Phet):
Aims of the activity:
1. Understand the importance of Einstein’s interpretation of this famous experiment.
2. Understand how this experiment provided evidence for the “particle” nature of light.
3. Use the scientific method appropriately in the lab design.
4. Analyse and interpret graphs that are generated.
Activity
This simulation allows you to “see” the photons bombarding the metal plate as well as the electrons that come off it. There are several factors to be studied:
1. The wavelength of the photons;
2. The intensity of the photons;
3. The type of metal plate being used.
We will break this down into 3 tests:
| Test | Independent Variable | Dependent Variable | Controls |
| Test 1 – Wavelength | Wavelength | Stopping Voltage | Intensity; type of metal |
| Test 2 – Intensity | Intensity | Stopping Voltage | Wavelength; type of metal |
| Test 3 – Type of metal plate | Same as Test 1 except you will use a different metal |
Hints about the simulation and lab
- You can either use the sliders to change the voltage, intensity, and wavelength, or you may manually enter the values. Manually will give you better control.
- Under Options, select “show photons”.
Test 1: Wavelength vs. Stopping Voltage
Select 5 different wavelengths. Use sodium as the metal. Be sure to include one UV wavelength. You will need to make the voltage as small as possible (negative) to create the least current. Make your intensity 100%. Be aware that some wavelengths will not cause any electron ejection. If that happens, you have to pick a shorter wavelength.
Test 1 results: You need to record wavelengths and stopping voltages. Calculate the frequencies of each wavelength, and calculate the kinetic energies due to the stopping voltages. Graph KE vs. frequency and record the slope and x and y-intercept values.
Test 2: Intensity vs. Stopping Voltage
Pick a wavelength in the violet/blue region to start. Use Sodium as the metal. Start with 100% intensity and find the lowest negative voltage. necessary to get a current.
Repeat at 3 other intensities with the same wavelength. (Make significant changes in the intensity. 100 to 90 is NOT significant).
Test 2 results: You need to record the stopping voltage at each intensity. If there appears to be a trend, you should graph this data.
Test 3: Different metals
You will do the same experiment as Test 1, except that you should select a different metal. Try to find 5 different wavelengths that give you results.
Test 3 results: You should do the same thing as Test 1.
Analysis/Conclusions:
Test 1 and 2: Use the Internet to find the work function for your metal. Calculate a percentage error. (Note: you will probably find the results in electron Volts. If so, to convert to Joules simply multiply by 1.6E-19.) Was your slope consistent with the actual value of h?
Test 2: What (if any) is the relationship between the intensity of the light and the stopping voltage?
Questions for further thought: you should do this.
1. In typical mechanical waves such as sound and water, what is the energy of the wave proportional to? For example, how do you recognize a high energy wave at the beach?
2. How do the results of this experiment seem to demonstrate that light energy is carried a different way?
3. Imagine 2 electrons on a plate of sodium metal. One electron is found right on the surface of the metal. The other electron is buried several layers below. A. Which electron needs the least energy to escape? Why? B. Which electron will have the greater KE when it absorbs the energy from a photon and escapes? Why? C. Which electron is more likely to overcome the electric field to reach the other metal? Why?
4. For sodium, even at 100% intensity, a green photon with a wavelength of 557 nm will never eject any electrons, but at 1% intensity, a UV photon at 235 nm always knocks out an electron. Einstein’s interpretation of this event (or one just like it) earned him a Nobel prize. It’s your turn. Why does this result clearly indicate particle-like properties of light (which Einstein referred to photons)?