The photoelectric effect is when light falls on/shines on a material and electrons are released from the material. The electrons released are known as photoelectrons.
Who discovered the photoelectric effect?
The photoelectric effect was discovered by the German physicist Heinrich Rudolf Hertz, in 1887. Einstein did not discover the photoelectric effect but in 1905 explained the observations, for which he later won the Nobel Prize. The phenomenon could not be explained using classical physics, which described light as electromagnetic waves.

Instead, Einstein used a particle theory of light (corpuscular) in which every particle of light, called a photon, carries a certain fixed amount of energy (or quanta) that depends on the frequency of light. Using these hypotheses, Einstein was able to explain all observations of the photoelectric effect.
How the photoelectric effect works.
The photoelectric effect is only observed above a minimum frequency, known as threshold frequency, fo. Below this, no photoelectrons are ejected. This is because each photon needs a minimum amount of energy to release a photoelectron from the metal.
This minimum photon energy required to emit an electron is dependent on the metal and is called the work function, W.
Photon energy is dependent on the frequency of the radiation according to the equation:
Ep= hf
Where Ep is photon energy (Joules), h is Planck constant, 6.63 x 10-34 Js and f is the frequency (Hz)
The photon energy must be at least equal to the work function for electrons to be released.
hfo = W
Photoelectrons are emitted when hf is greater or equal to W.
Below the threshold frequency, no photoelectrons are emitted because hf < W.
Photoelectric effect equation
When radiation of sufficiently high frequency, f, is incident on a metal surface of work function, W. The photon, of energy Ep, is absorbed by the electron. This electron leaves with kinetic energy Kmax = Ep –W
The photon energy Ep = hf and the maximum electron kinetic energy = ½ mv2.
½ mv2 = hf – W
What does the photoelectric effect prove?
The photoelectric effect proves that energy is quantised. This means that energy arrives in ‘lumps’ known as quanta. These lumps or packets of energy are called photons. This contradicts the long accepted wave model, where light is considered as an electromagnetic wave, with energy arriving continuously. The photon model was Einstein’s explanation of the photoelectric effect.
Why can’t photoelectric effect be explained by the wave model?
If the incident light has a frequency lower than the threshold frequency then no photoelectrons are emitted, even if the brightness of the light is cranked right up. This shows that energy is not transferred continuously as predicted by the wave model of light.
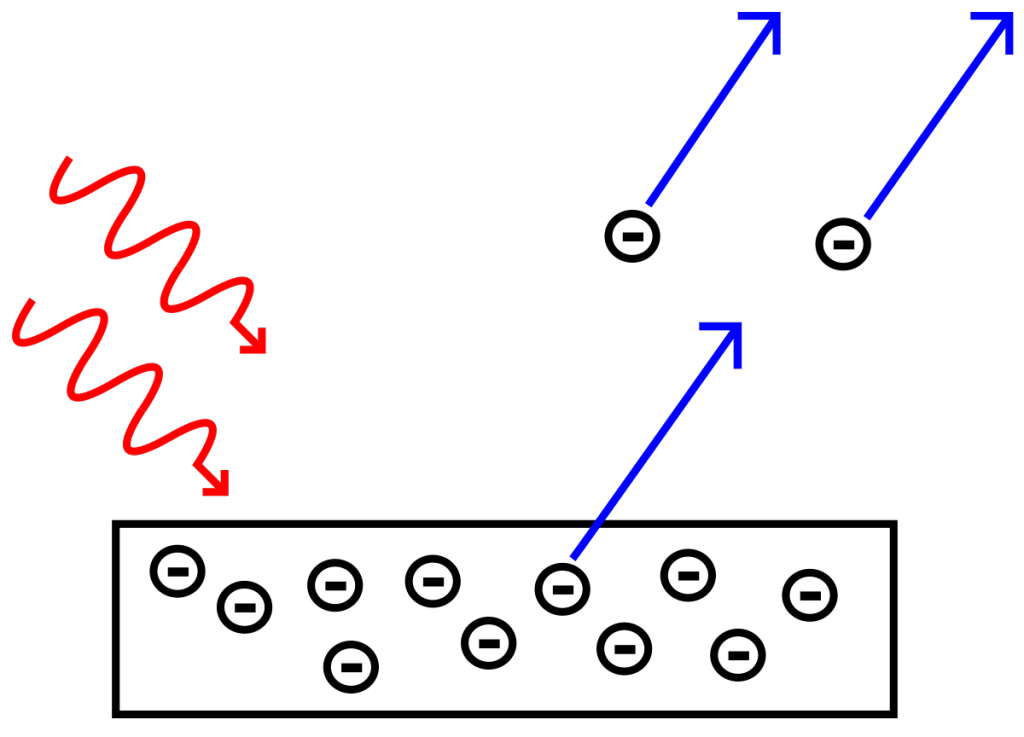
Light frequency above the threshold frequency. Photoelectrns emitted 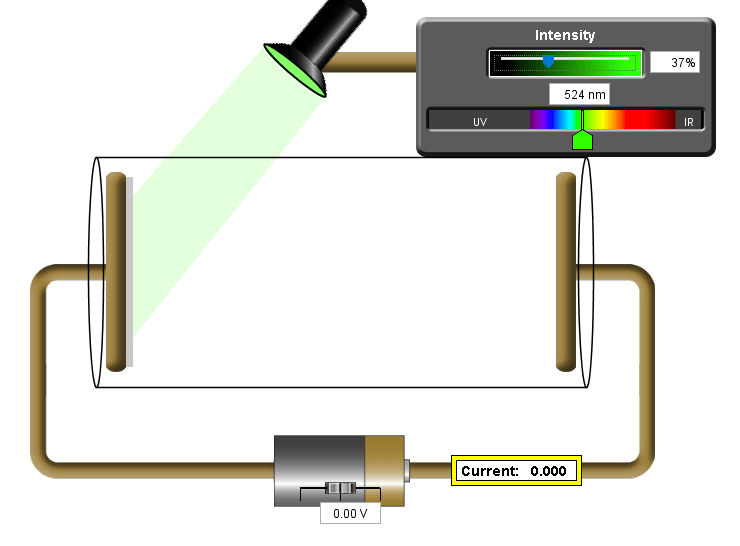
Light frequency below the threshold frequency. No photoelectrons emitted
With the wave model, it should be possible to increase the intensity of the light or wait for a period of time, until sufficient energy is absorbed by the material to release electrons. This does not happen. There is essentially no delay between absorption of the radiant energy and the emission of photoelectrons.
The wave model, which dates back to Huygens in late 1600’s, is still very successful at explaining phenomena such as diffraction (spreading out of light) and interference (overlapping effect of light causing patches of increased/decreased intensity). However, the wave theory cannot explain photoelectric effect.
The maximum kinetic energy of the released electrons does not vary with the intensity of light, as was expected with wave theory, but was instead found to increase with the frequency of light. What the light intensity did was to determine the number of electrons released from the matter, measured as an electric current.
How does photoelectric effect show light is a particle?
For the photon to eject an electron, there must be enough energy in a single photon. You can’t use lots and lots of lower energy photons. This proves that photons are discrete packets of energy. In other words, light exhibits a particle nature, where energy arrives in lumps or quanta. Modern physicists have concluded that both the particle theory an wave theory are simplified explanations for a complex behaviour, known as wave-particle
duality.
Which metals exhibit photoelectric effect?
All metals can show the photoelectric effect. Different materials have different workfunctions and threshold frequencies. This means the minimum photon energy required to overcome the workfunction will depend on the material.
Most metals have threshold frequencies in the ultraviolet region of the electromagnetic spectrum. Zinc, for example, has a work function of 4.3eV, which corresponds to a threshold frequency of approximately 1 x 1015Hz (wavelength =287nm), which is in the ultraviolet range.
Can non-metals show photoelectric effect?
Non-metals can show the photoelectric effect. With atoms that hold their electrons tightly, a photon is much less likely to be absorbed and release an electron. This is because a greater photon energy is needed to make it happen. The Photoelectric effect can still happen in non-metals, but it is
more likely and possible for a wider range of frequencies in a metal.
Photoelectric effect experiment
Metals have a ‘sea’ of free electrons. It is these spare electrons which are able to be dislodged by passing photons, provided that the photon has sufficient energy. That said, some non-metals, mostly in the metalloid group, can exhibit weak photoelectric effects,
If the energy of the inbound photons is high enough. The photons might need to have a very high frequency, perhaps in the X-ray region. Compounds such as water, are MUCH less likely to show any photoelectric effect, because the electrons are all neatly paired off and bound up in shared electron shells and so on (covalent bonds), reducing the chance of
ejecting an electron.
In the classic photoelectric effect experiment, a stopping potential is applied between the metal surface emitting photoelectrons and the target plate. The stopping potential is set so that the electrons are stopped in their tracks. From this the electron energy ½ mv2 is assumed to be equal to the work done in stopping them, eVs(e=1.6 x 10-19C, Vs is the stopping potential applied).
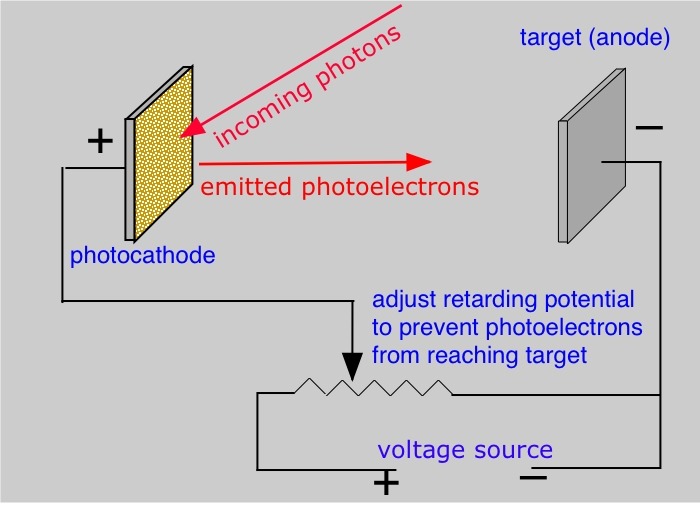
The photoelectric effect equation can be written as
eVs = hf – W
If the experiment is repeated with light of various frequencies above the threshold frequency, a graph is then plotted of electron KE (eVs) versus frequency. The line is extrapolated to find the x and y intercept values.
The gradient of the line gives a value of h (Planck constant). The x intercept is fo , the threshold frequency. The y intercept is –W (-work function).
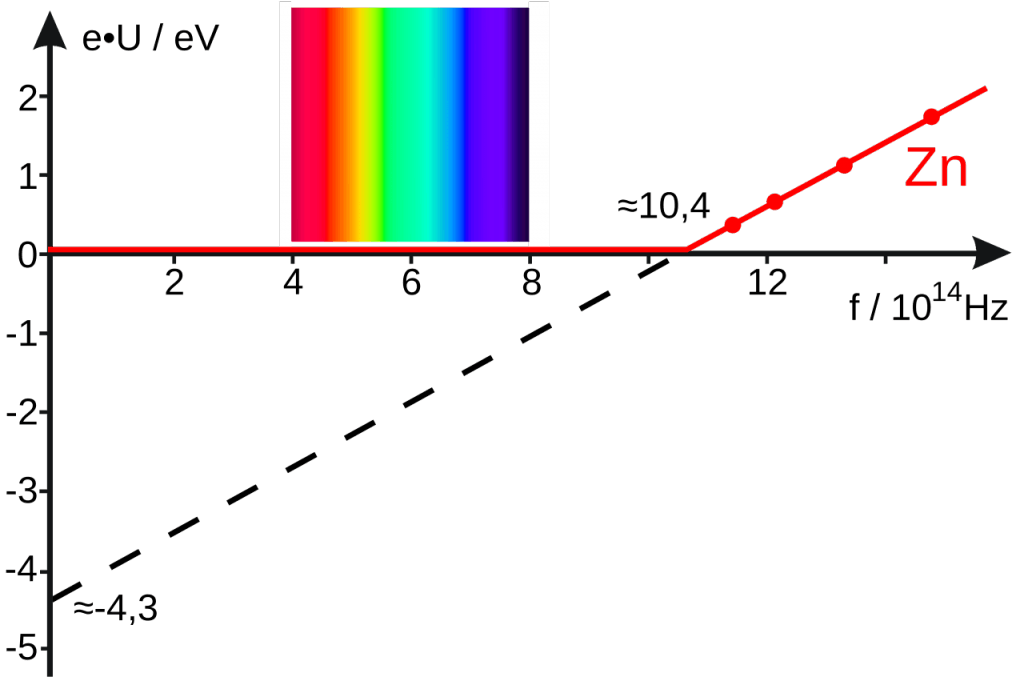
The graph shows values for zinc. A similar graph is obtained for all metals with the same gradient (Planck constant) and different values for fo and W.
Photoelectric effect in solar cells
Solar Panels usually use semiconductor materials such as silicon or gallium arsenide. The photoelectric effect is observed when a solar cell converts light to electrical energy.

Photons can be absorbed by the semiconductor material in a solar cell. This results in the excitation of an electron from the valence band to the conduction band. Semiconductors, such as silicon, have a different structure to metals. We can’t use the work function in the same way to
calculate minimum photon energy as we do for metals. Essentially, semiconductors have a filled valence band and an empty conduction band. The photon energy is needed to make an electron move into the conduction band.
It is more difficult to observe the photoelectric effect in insulators, where the electrons are more tightly bound.
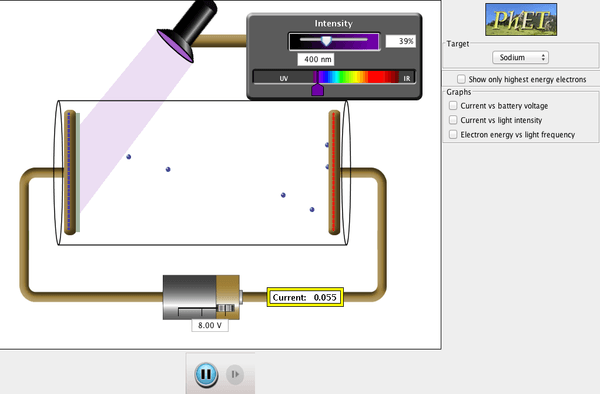
The photoelectric effect is best simulated using the Phet simulation
Now it’s your turn – can you find the mystery metal?
Text
The text in this article is licensed under the Creative Commons-License Attribution 4.0 International (CC BY 4.0).
This means you’re free to copy, share and adapt any parts (or all) of the text in the article, as long as you give appropriate credit and provide a link/reference to this page.
You don’t need my permission to copy the article; just include a link/reference back to this page. You can use it freely (with some kind of link), and I’m also okay with people reprinting in publications like books, blogs, course-material, papers, wikipedia and presentations (with clear attribution).
Download PDF of this article
Go to photoelectric effect simulation activity using the Phet simulator




Love the PE resource but units of h should be Js not JS! Also x-ray not x-rays region?? Sorry – I have a first in pendantry!
Hi David, That’s really helpful, well spotted and thank you. I can change these quite easily. Regards, Tim
The photoelectric effect occurs when an atom absorbs the energy of a photon and an electron is emitted. Some energy is used to liberate the electron, the rest into electron kinetic energy. The same thing cannot happen when light interacts with a free electron. A free electron cannot absorb all the energy of a photon and simultaneously conserve linear momentum.
and, instead, part of the photon energy is given to the electron and a photon of lower energy is scattered. This is known as the Compton effect!